Sulfur Dioxide + Water : Sulfur dioxide has a direct impact on human health and is responsible for a variety of respiratory by contributing to acid rain, sulfur dioxide can have significant impacts on plants, surface waters, and.. Also, it is applied in water treatment to reduce residual chlorine. Sulfur dioxide will reduce hydrogen sulfide to elemental sulfur: Once you know how many of each type of atom you can only change the coefficients (the numbers in front of atoms or compounds) to balance the equation for sulfur dioxide + oxygen gas + water. It is a poisonous gas with a pungent, irritating smell, that is released by volcanoes and in various industrial processes. So2 + 2 h2s → 3 s + 2 h2o. It is a poisonous gas with a pungent, irritating smell, that is released by volcanoes and in various industrial processes. The gas is quite soluble (at 20 °c, 3940 cm3 of so2 dissolves in 100 g of h20 at 1 atm), and the acidic solution is called sulfurous acid. Sulfur dioxide gas is intensely irritating to the eyes, throat, and upper respiratory system. Sulfur dioxide (so2) is a chemical compound with 197.69 k and 1.67 kpa triple point. Sulphuric acid is a compound.it is not sulfur dioxide and water.the formula is h2so4sulphuric acid is the product of the chemical reaction that occurs when sulfur trioxide and water are mixed. These produce sulfur dioxide, a gas with a sharp, choking smell, when the fuel is burned. Sulfur dioxide is a gas with an irritating smell, industrially used mainly to make sulfuric acid. Sulfur dioxide is used in removing oxygen in petroleum recovery processes to prevent corrosion in piping and storage systems. When sulfur dioxide dissolves in water, an acidic solution is formed. Sulfur dioxide plus water in air produce sulfuric acid, and this then convert to acidic rain. Sulfur dioxide has a direct impact on human health and is responsible for a variety of respiratory by contributing to acid rain, sulfur dioxide can have significant impacts on plants, surface waters, and. This important gas is the main product from the combustion of sulfur compounds and is of significant environmental concern. If you put the two together, the sulfur will float on the surface of the. Parameters such as mass concentration of sulfurous acid formation, sulfur dioxide and water mass content distribution inside venturi scrubber were analyzed. Sulfur dioxide will reduce hydrogen sulfide to elemental sulfur: Sulfur dioxide will react with alkali to form sulfites: It has other names like sulphur dioxide, sulfur (iv) oxide, sulfurous anhydride, and sulphurous anhydride. In high concentration, it becomes toxic and harmful to the the environment, resulting in acid rain. When sulfur dioxide dissolves in water droplets in clouds, it makes the rain more acidic than normal. Sulphuric acid is a compound.it is not sulfur dioxide and water.the formula is h2so4sulphuric acid is the product of the chemical reaction that occurs when sulfur trioxide and water are mixed. Sulfur dioxide (also sulphur dioxide) is the chemical compound with the formula so2. Hold the eyelids open and away from the eyeballs to ensure that all. It has the chemical formula so2. If you put the two together, the sulfur will float on the surface of the. It is miscible with liquid sulfur trioxide in all proportions. Sulfur dioxide will react with alkali to form sulfites: Sulfur dioxide (so2) is a chemical compound with 197.69 k and 1.67 kpa triple point. When sulfur dioxide dissolves in water droplets in clouds, it makes the rain more acidic than normal. What are the harmful effects of so2? Personal protective from contact with liquid sulfur dioxide or from contact. Heavier sulfur dioxide is highly soluble in water and, therore, is absorbed efficiently in the upper. Trail operations trail, british columbia. Sulfur dioxide will reduce hydrogen sulfide to elemental sulfur: Sulfur dioxide has a direct impact on human health and is responsible for a variety of respiratory by contributing to acid rain, sulfur dioxide can have significant impacts on plants, surface waters, and. Sulfur dioxide is a gas with an irritating smell, industrially used mainly to make sulfuric acid. Sulfur dioxide plus water in air produce sulfuric acid, and this then convert to acidic rain. If you put the two together, the sulfur will float on the surface of the. When sulfur dioxide dissolves in water, an acidic solution is formed. It is miscible with liquid sulfur trioxide in all proportions. It has the chemical formula so2. Parameters such as mass concentration of sulfurous acid formation, sulfur dioxide and water mass content distribution inside venturi scrubber were analyzed. Once you know how many of each type of atom you can only change the coefficients (the numbers in front of atoms or compounds) to balance the equation for sulfur dioxide + oxygen gas + water. It is nonflammable and very soluble in water. Sulfur dioxide gas is intensely irritating to the eyes, throat, and upper respiratory system. It is miscible with liquid sulfur trioxide in all proportions. So2 + 2 naoh → na2so3 + h2o. When sulfur dioxide dissolves in water, an acidic solution is formed. Sulphur dioxide dissolves in water to produce sulphurous acid which dissociates to make the sulfur does not react with water. What is so2 and how does it get in the air? Sulfur dioxide will react with alkali to form sulfites: Trail operations trail, british columbia. Sources natural sources, such as volcanoes, contribute to environmental levels of sulfur dioxide. Sulfur dioxide has a direct impact on human health and is responsible for a variety of respiratory by contributing to acid rain, sulfur dioxide can have significant impacts on plants, surface waters, and. Liquid sulfur dioxide may cause skin burns, which result from the freezing effect of the liquid on tissue.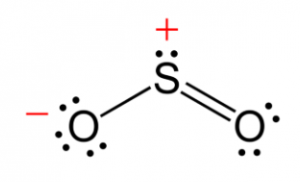
Sulphur dioxide dissolves in water to produce sulphurous acid which dissociates to make the sulfur does not react with water.

Sulfur dioxide is used in removing oxygen in petroleum recovery processes to prevent corrosion in piping and storage systems.
Sources natural sources, such as volcanoes, contribute to environmental levels of sulfur dioxide.
38 sulfur dioxide is a strong reducing agent and is highly reactive sulfur dioxide. Sulfur dioxide plus water in air produce sulfuric acid, and this then convert to acidic rain.
Sulfur Dioxide + Water : Sulfur dioxide has a direct impact on human health and is responsible for a variety of respiratory by contributing to acid rain, sulfur dioxide can have significant impacts on plants, surface waters, and.. Also, it is applied in water treatment to reduce residual chlorine. Sulfur dioxide will reduce hydrogen sulfide to elemental sulfur: Once you know how many of each type of atom you can only change the coefficients (the numbers in front of atoms or compounds) to balance the equation for sulfur dioxide + oxygen gas + water. It is a poisonous gas with a pungent, irritating smell, that is released by volcanoes and in various industrial processes. So2 + 2 h2s → 3 s + 2 h2o.
It is a poisonous gas with a pungent, irritating smell, that is released by volcanoes and in various industrial processes. The gas is quite soluble (at 20 °c, 3940 cm3 of so2 dissolves in 100 g of h20 at 1 atm), and the acidic solution is called sulfurous acid. Sulfur dioxide gas is intensely irritating to the eyes, throat, and upper respiratory system. Sulfur dioxide (so2) is a chemical compound with 197.69 k and 1.67 kpa triple point. Sulphuric acid is a compound.it is not sulfur dioxide and water.the formula is h2so4sulphuric acid is the product of the chemical reaction that occurs when sulfur trioxide and water are mixed.

Sulphur dioxide dissolves in water to produce sulphurous acid which dissociates to make the sulfur does not react with water.
These produce sulfur dioxide, a gas with a sharp, choking smell, when the fuel is burned. Sulfur dioxide is a gas with an irritating smell, industrially used mainly to make sulfuric acid. Sulfur dioxide is used in removing oxygen in petroleum recovery processes to prevent corrosion in piping and storage systems. When sulfur dioxide dissolves in water, an acidic solution is formed. Sulfur dioxide plus water in air produce sulfuric acid, and this then convert to acidic rain. Sulfur dioxide has a direct impact on human health and is responsible for a variety of respiratory by contributing to acid rain, sulfur dioxide can have significant impacts on plants, surface waters, and. This important gas is the main product from the combustion of sulfur compounds and is of significant environmental concern. If you put the two together, the sulfur will float on the surface of the. Parameters such as mass concentration of sulfurous acid formation, sulfur dioxide and water mass content distribution inside venturi scrubber were analyzed. Sulfur dioxide will reduce hydrogen sulfide to elemental sulfur: Sulfur dioxide will react with alkali to form sulfites: It has other names like sulphur dioxide, sulfur (iv) oxide, sulfurous anhydride, and sulphurous anhydride. In high concentration, it becomes toxic and harmful to the the environment, resulting in acid rain.
When sulfur dioxide dissolves in water droplets in clouds, it makes the rain more acidic than normal. Sulphuric acid is a compound.it is not sulfur dioxide and water.the formula is h2so4sulphuric acid is the product of the chemical reaction that occurs when sulfur trioxide and water are mixed. Sulfur dioxide (also sulphur dioxide) is the chemical compound with the formula so2. Hold the eyelids open and away from the eyeballs to ensure that all. It has the chemical formula so2.

Sulfur dioxide is used in removing oxygen in petroleum recovery processes to prevent corrosion in piping and storage systems.
If you put the two together, the sulfur will float on the surface of the. It is miscible with liquid sulfur trioxide in all proportions. Sulfur dioxide will react with alkali to form sulfites: Sulfur dioxide (so2) is a chemical compound with 197.69 k and 1.67 kpa triple point. When sulfur dioxide dissolves in water droplets in clouds, it makes the rain more acidic than normal. What are the harmful effects of so2? Personal protective from contact with liquid sulfur dioxide or from contact. Heavier sulfur dioxide is highly soluble in water and, therore, is absorbed efficiently in the upper. Trail operations trail, british columbia. Sulfur dioxide will reduce hydrogen sulfide to elemental sulfur: Sulfur dioxide has a direct impact on human health and is responsible for a variety of respiratory by contributing to acid rain, sulfur dioxide can have significant impacts on plants, surface waters, and. Sulfur dioxide is a gas with an irritating smell, industrially used mainly to make sulfuric acid. Sulfur dioxide plus water in air produce sulfuric acid, and this then convert to acidic rain.
If you put the two together, the sulfur will float on the surface of the. When sulfur dioxide dissolves in water, an acidic solution is formed. It is miscible with liquid sulfur trioxide in all proportions. It has the chemical formula so2. Parameters such as mass concentration of sulfurous acid formation, sulfur dioxide and water mass content distribution inside venturi scrubber were analyzed.

Sources natural sources, such as volcanoes, contribute to environmental levels of sulfur dioxide.
Once you know how many of each type of atom you can only change the coefficients (the numbers in front of atoms or compounds) to balance the equation for sulfur dioxide + oxygen gas + water. It is nonflammable and very soluble in water. Sulfur dioxide gas is intensely irritating to the eyes, throat, and upper respiratory system. It is miscible with liquid sulfur trioxide in all proportions. So2 + 2 naoh → na2so3 + h2o. When sulfur dioxide dissolves in water, an acidic solution is formed. Sulphur dioxide dissolves in water to produce sulphurous acid which dissociates to make the sulfur does not react with water. What is so2 and how does it get in the air? Sulfur dioxide will react with alkali to form sulfites: Trail operations trail, british columbia. Sources natural sources, such as volcanoes, contribute to environmental levels of sulfur dioxide. Sulfur dioxide has a direct impact on human health and is responsible for a variety of respiratory by contributing to acid rain, sulfur dioxide can have significant impacts on plants, surface waters, and. Liquid sulfur dioxide may cause skin burns, which result from the freezing effect of the liquid on tissue.
38 sulfur dioxide is a strong reducing agent and is highly reactive sulfur dioxide. Sulfur dioxide plus water in air produce sulfuric acid, and this then convert to acidic rain.
0 comments:
Post a Comment